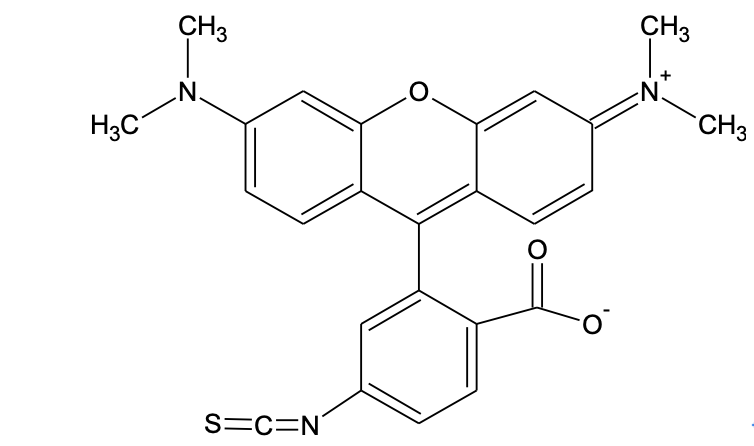
Fluorescent Dye Isothiocyanates
Isothiocyanates form thioureas upon reaction with amines. It is proven that some thiourea products (in particular, the conjugates from a-amino acids/peptides/proteins) are much less stable than the conjugates that are prepared from the corresponding succinimidyl esters. It has been reported that antibody conjugates prepared from fluorescein isothiocyanates deteriorate over time. We strongly recommend that you use succinimidy esters for your conjugations whenever possible. There are few factors that need be considered when SE compounds are used for conjugation reaction:
1). Solvents:For the most part, reactive dyes are hydrophobic molecules and should be dissolved in anhydrous dimethylformamide (DMF) or dimethylsulfoxide (DMSO).
2). Reaction pH:The labeling reactions of amines with isothiocyanates are strongly pH dependent. Isothiocynate reagents react with non-protonated aliphatic amine groups, including the terminal amines of proteins and the e-amino groups of lysines. Protein modifications by isothiocyanates may require a pH 9.0-10.0 for optimal conjugations.
3). Reaction Buffers:Buffers that contain free amines such as Tris and glycine must be avoided when using an amine-reactive reagent. Ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation must also be removed before performing dye conjugations. High concentrations of nucleophilic thiol compounds should also be avoided because they may react with the labeling reagent to form unstable intermediates that could destroy the reactive dye.
4). Reaction Temperature:Isothiocynate conjugations are usually done at room temperature. However, either elevated or reduced temperature may be required for a particular labeling reaction.
6-TRITC; R isomer [Tetramethylrhodamine-6-isothiocyanate]
| Features and Biological Applications6-TRITC (also called R isomer) is the other isomer of the TRITC labeling reagent that is widely used in preparing bioconjugates of proteins and nucleic acids. Complimentary to 5-TRITC, the 6-isomer is predominantly used in labeling nucleotides and nucleic acids.Cautions must b exercised for the storage of TRITC conjugates.
|
References
1. Kahn E, et al.(1999). Confocal-multilabeling, ultrasensitive TUNEL analysis of DNA breaks in individual cells. Anal Quant Cytol Histol21, 1-7.
2. Nederlof PM, et al.(1992). Fluorescence ratio measurements of double-labeled probes for multiple in situ hybridization by digital imaging microscopy. Cytometry13, 839-45.
3. Meadows, D.L., et al., Determining the extent of labeling for tetramethylrhodamine protein conjugates.J Immunol Methods1991, 143, 263-72.
特别提醒:
1) 本公司所有产品仅限于专业人员用于生命科学研究,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅。
2) 本公司所有产品必须由合格专业技术人员操作同时佩戴口罩/手套/实验服并遵守生物实验室安全操作规程!
| Name | 6-TRITC; R isomer [Tetramethylrhodamine-6-isothiocyanate] | ||
|---|---|---|---|
| CAT# | 417-5mg | CAS# | 80724-20-5 |
| Storage# | -20°C Sealed & desiccated & Minimized light exposure | Shelf Life# | 24 months |
| Ex(nm)# | 543 | Em(nm)# | 571 |
| MW# | 443.52 | Solvent# | DMF or DMSO |
| Name | 6-TRITC; R isomer [Tetramethylrhodamine-6-isothiocyanate] |
|---|---|
| CAT# | 417-5mg |
| CAS# | 80724-20-5 |
| Storage# | -20°C Sealed & desiccated & Minimized light exposure |
| Shelf Life# | 24 months |
| Ex(nm)# | 543 |
| Em(nm)# | 571 |
| MW# | 443.52 |
| Solvent# | DMF or DMSO |

![6-TRITC; R isomer [Tetramethylrhodamine-6-isothiocyanate]详细说明书](/Public/Home/images/pic52.png) 详细说明书
详细说明书![6-TRITC; R isomer [Tetramethylrhodamine-6-isothiocyanate]技术资料](/Public/Home/images/pic53.png) 技术资料
技术资料