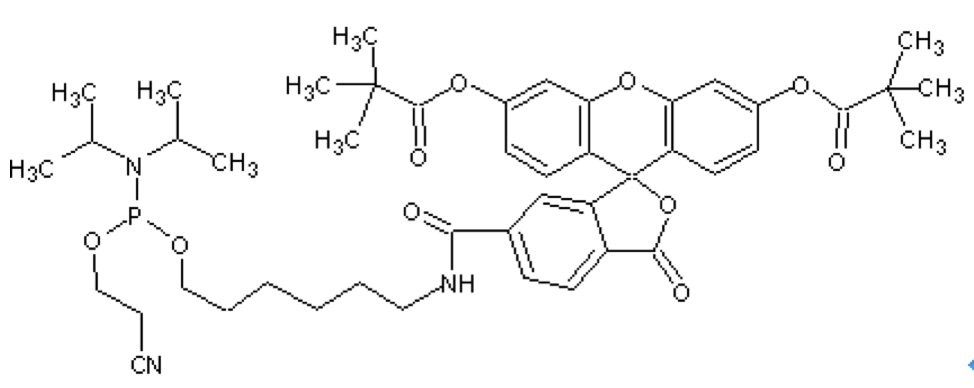
5’-Fluorescein phosphoramidite
Cat# | Size | Price | MW | Abs | Em | Soluble in | Storage |
6020 | 1 g | $950 | 843.94 | 494 nm | 522 nm | MeCN | F/D/L |
|
Features and Biological ApplicationsThere are several ways of labeling an oligonucleotide with fluorescein. The choice of label is diversified further, depending on the spectral requirements. 5’-fluorescein-CE phosphoramidite, derived from the single isomer 6-carboxyfluorescein, 5’-hexachlorofluorescein-CE phosphoramidite (HEX) and 5’-tetrachlorofluorescein-CE phosphoramidite (TET) can all be used to efficiently label an oligonucleotide at the 5’-end. Labeling the 3’-end of an oligo with fluorescein can be achieved using 3’-Fluorescein CPGs. Standard cleavage and deprotection with ammonium hydroxide liberates the fluorescein-labeled oligo when using any of these supports. |
References
1. Browne KA. (2005) Sequence-specific, self-reporting hairpin inversion probes. J Am Chem Soc, 127, 1989.
2.Dobson N, McDowell DG, French DJ, Brown LJ, Mellor JM, Brown T. (2003) Synthesis of HyBeacons and dual-labelled probes containing 2'-fluorescent groups for use in genetic analysis. Chem Commun (Camb), 1234.
3. Hagmar P, Bailey M, Tong G, Haralambidis J, Sawyer WH, Davidson BE. (1995) Synthesis and characterisation of fluorescent oligonucleotides. Effect of internal labelling on protein recognition. Biochim Biophys Acta, 1244, 259.
4. Hakala H, Virta P, Salo H, Lonnberg H. (1998) Simultaneous detection of several oligonucleotides by time-resolved fluorometry: the use of a mixture of categorized microparticles in a sandwich type mixed-phase hybridization assay. Nucleic Acids Res, 26, 5581.
5. Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W, McGee DP, Husar GM, Leuck M, Anziano DJ, Sebesta DP. (2001) Diels--Alder bioconjugation of diene-modified oligonucleotides. J Org Chem, 66, 5352.
6. Kim SJ, Bang EK, Kwon HJ, Shim JS, Kim BH. (2004) Modified oligonucleotides containing lithocholic acid in their backbones: their enhanced cellular uptake and their mimicking of hairpin structures. Chembiochem, 5, 1517.
7. Kutyavin IV, Lokhov SG, Afonina IA, Dempcy R, Gall AA, Gorn VV, Lukhtanov E, Metcalf M, Mills A, Reed MW, Sanders S, Shishkina I, Vermeulen NM. (2002) Reduced aggregation and improved specificity of G-rich oligodeoxyribonucleotides containing pyrazolo[3,4-d]pyrimidine guanine bases. Nucleic Acids Res, 30, 4952.
8. Lee SP, Censullo ML, Kim HG, Knutson JR, Han MK. (1995) Characterization of endonucleolytic activity of HIV-1 integrase using a fluorogenic substrate. Anal Biochem, 227, 295.
9. Meyer KL, Hanna MM. (1996) Synthesis and characterization of a new 5-thiol-protected deoxyuridine phosphoramidite for site-specific modification of DNA. Bioconjug Chem, 7, 401.
NOTE: Always wear lab coats, gloves and goggles when working with our products although they are low-risk chemicals for R&D only.
| Name | 5’-Fluorescein phosphoramidite | ||
|---|---|---|---|
| CAT# | 6020-100mg | CAS# | 204697-37-0 |
| Storage# | F/D/L | Shelf Life# | 12 months |
| Ex(nm)# | 494 | Em(nm)# | 522 |
| MW# | 843.94 | Solvent# | MeCN |
| Name | 5’-Fluorescein phosphoramidite |
|---|---|
| CAT# | 6020-100mg |
| CAS# | 204697-37-0 |
| Storage# | F/D/L |
| Shelf Life# | 12 months |
| Ex(nm)# | 494 |
| Em(nm)# | 522 |
| MW# | 843.94 |
| Solvent# | MeCN |

 Specification
Specification Support
Support