Fluorescent Dye Carboxylic Acids and Their Succinimidyl Esters
Succinimidyl esters are proven to be the best reagents for amine modifications because the amide bonds that are formed are essentially identical to, and as stable as the natural peptide bonds. These reagents are generally stable and show good reactivity and selectivity with aliphatic amines. There are few factors that need be considered when SE compounds are used for conjugation reaction:
1). Solvents:For the most part, reactive dyes are hydrophobic molecules and should be dissolved in anhydrous dimethylformamide (DMF) or dimethylsulfoxide (DMSO).
2). Reaction pH:The labeling reactions of amines with succinimidyl esters are strongly pH dependent. Amine-reactive reagents react with non-protonated aliphatic amine groups, including the terminal amines of proteins and the e-amino groups of lysines. Thus amine acylation reactions are usually carried out above pH 7.5. Protein modifications by succinimidyl esters can typically be done at pH 7.5-8.5, whereas isothiocyanates may require a pH 9.0-10.0 for optimal conjugations.
3).Reaction Buffers:Buffers that contain free amines such as Tris and glycine and thiol compounds must be avoided when using an amine-reactive reagent. Ammonium salts (such as ammonium sulfate and ammonium acetate) that are widely used for protein precipitation must also be removed (such as viadinlysis) before performing dye conjugations.
4). Reaction Temperature:Most conjugations are done at room temperature. However, either elevated or reduced temperature may be required for a particular labeling reaction.
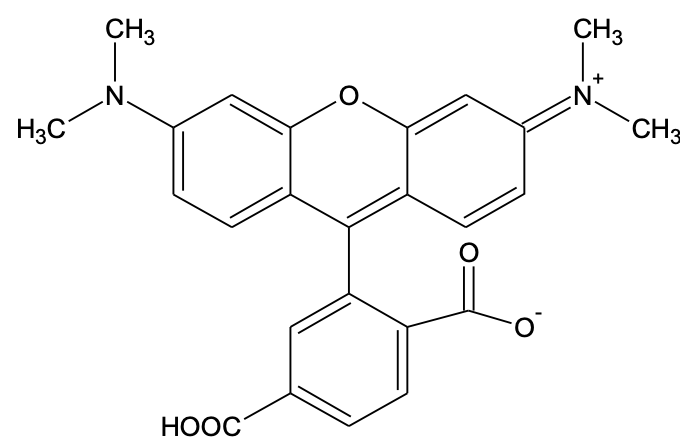
6-TAMRA [6-Carboxytetramethylrhodamine]
| Features and Biological Applications6-TAMRA is the other purified single isomer of 5(6)-TAMRA. It is predominantly used for nucleotide labeling. It is also used in fluorescence in situhybridization (FISH). |
References
1. Hsu TM, et al. (2001). Genotyping single-nucleotide polymorphisms by the invader assay with dual-color fluorescence polarization detection. Clin Chem47, 1373-7.
2. Schutz E, et al. (2000). Genotyping of eight thiopurine methyltransferase mutations: three-color multiplexing, two-color/shared anchor and fluorescence-quenching hybridization probe assays based on thermodynamic nearest-neighbor probe design. Clin Chem46, 1728-37.
3. Lyttle, M.H., et al., A tetramethyl rhodamine (tamra) phosphoramidite facilitates solid-phase-supported synthesis of 5'-tamra DNA.J Org Chem2000, 65, 9033-8.
NOTE: Always wear lab coats, gloves and goggles when working with our products although they are low-risk chemicals for R&D only.
| Name | 6-TAMRA | ||
|---|---|---|---|
| CAT# | 366-10mg;366-100mg | CAS# | 91809-67-5 |
| Storage# | −20°C and desiccated | Shelf Life# | 12个月 |
| Ex(nm)# | 541 | Em(nm)# | 568 |
| MW# | 430.45 | Solvent# | DMSO |
| Name | 6-TAMRA |
|---|---|
| CAT# | 366-10mg;366-100mg |
| CAS# | 91809-67-5 |
| Storage# | −20°C and desiccated |
| Shelf Life# | 12个月 |
| Ex(nm)# | 541 |
| Em(nm)# | 568 |
| MW# | 430.45 |
| Solvent# | DMSO |

 Specification
Specification Support
Support